3-Chloroaniline: applications and degradation pathways
General Description
3-Chloroaniline is a chemical compound used in the production of dyes, pharmaceuticals, and pesticides. Its presence in industrial wastewater can negatively impact biological wastewater treatment systems. Studies have shown that exposure to 3-chloroaniline can decrease the efficiency of chemical oxygen demand (COD) and NH4+-N removal in sequencing batch reactors (SBR). However, over time, these removal efficiencies can recover. The shock loading of 3-chloroaniline also affects enzyme activity and microbial communities in the reactor, leading to increased production of reactive oxygen species and changes in microbial diversity. Understanding the degradation pathways of 3-chloroaniline is important for environmental management. It can be biodegraded by specific bacteria, undergo photochemical degradation through sunlight exposure, or be degraded through advanced oxidation processes. These pathways help minimize its environmental impact and ensure ecosystem and human health safety.

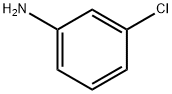
Figure 1. 3-Chloroaniline
Applications
3-Chloroaniline is widely used in various industrial processes, including the production of dyes, pharmaceuticals, and pesticides. However, its presence in industrial wastewater can have detrimental effects on biological wastewater treatment systems due to its potential biotoxicity. In a recent study, the application of 3-chloroaniline was evaluated in a sequencing batch reactor (SBR) used for wastewater treatment. The reactor's performance, nitrogen removal rate, microbial community, and enzymatic activity were assessed under transient 3-chloroaniline shock loading. The results showed that after a 24-hour exposure to 40 mg/L of 3-chloroaniline on day 9, the efficiency of chemical oxygen demand (COD) removal decreased from 90.71% on day 8 to 80.57% on day 11. Similarly, the removal efficiency of NH4+-N reduced from 98.96% on day 8 to 35.51% on day 12. However, over time, both COD and NH4+-N removal efficiencies gradually recovered to their normal values. Furthermore, the shock loading of 3-chloroaniline had a significant impact on the activities of key enzymes and microbial communities in the reactor. Additionally, the presence of 3-chloroaniline led to increased production of reactive oxygen species in the microbial community and the release of lactate dehydrogenase. Moreover, the shock loading affected the diversity and richness of the microbial community. These findings provide valuable insights into the effects of transient 3-chloroaniline shock on biological wastewater treatment systems. They contribute to the development of preventive measures and strategies to mitigate the adverse effects of 3-chloroaniline on bioreactor performance.1
Degradation pathways
3-Chloroaniline is an organic compound with the chemical formula C6H6ClN. However, its release into the environment can pose significant risks to human health and ecosystems due to its toxicity and persistence. Therefore, understanding the degradation pathways of 3-Chloroaniline is crucial for environmental management. There are several ways in which 3-Chloroaniline can be degraded. One common method is through biodegradation by microorganisms. Certain bacteria have the ability to utilize 3-Chloroaniline as a carbon and energy source. These microorganisms possess specific enzymes that break down the chemical bonds of 3-Chloroaniline, converting it into less harmful compounds. The process typically involves stepwise removal of the chlorine atom followed by further degradation of the resulting intermediates. Another degradation pathway is photochemical degradation. When exposed to sunlight, 3-Chloroaniline can undergo photolysis, a process in which the chemical bonds are broken by the energy from light. This results in the formation of reactive intermediates that react with other molecules in the environment, eventually leading to the complete degradation of 3-Chloroaniline. Additionally, advanced oxidation processes (AOPs) can be employed for the degradation of 3-Chloroaniline. AOPs involve the use of powerful oxidants such as ozone, hydrogen peroxide, or UV radiation to generate highly reactive species like hydroxyl radicals. These radicals can attack and break down the molecular structure of 3-Chloroaniline, transforming it into less toxic substances. In conclusion, 3-Chloroaniline can be degraded through various mechanisms, including biodegradation by microorganisms, photochemical degradation, and advanced oxidation processes. These degradation pathways play a crucial role in minimizing the environmental impact of 3-Chloroaniline and ensuring the safety of ecosystems and human health. 2, 3
Reference
1. Ma B, Zhao C, Li S, Gao M, She Z, Yu N, Guo L, Zhao Y, Jin C. Effects of transient 3-chloroaniline shock loading on the performance, microbial community and enzymatic activity of sequencing batch reactor. J Environ Manage. 2020 Mar 15;258:110017.
2. Seshan H, Santillan E, Constancias F, Chandra Segaran US, Williams RBH, Wuertz S. Metagenomics and metatranscriptomics suggest pathways of 3-chloroaniline degradation in wastewater reactors. Sci Total Environ. 2023 Aug 5;903:166066.
3. Zhang LL, He D, Chen JM, Liu Y. Biodegradation of 2-chloroaniline, 3-chloroaniline, and 4-chloroaniline by a novel strain Delftia tsuruhatensis H1. J Hazard Mater. 2010 Jul 15;179(1-3):875-882.
You may like
Related articles And Qustion
See also
Lastest Price from 3-Chloroaniline manufacturers

US $1.00/KG2025-04-21
- CAS:
- 108-42-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $30.00/KG2023-10-08
- CAS:
- 108-42-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20T
![3680-69-1 Properties of 4-Chloro-7H-pyrrolo[2,3-d]pyrimidineapplications of 4-Chloro-7H-pyrrolo[2,3-d]pyrimidinesafety of 4-Chloro-7H-pyrrolo[2,3-d]pyrimidine](/NewsImg/2024-02-15/6384361474941818812500588.jpg)
![3680-69-1 Properties of 4-Chloro-7H-pyrrolo[2,3-d]pyrimidineapplications of 4-Chloro-7H-pyrrolo[2,3-d]pyrimidinesafety of 4-Chloro-7H-pyrrolo[2,3-d]pyrimidine](/NewsImg/2023-09-25/6383126719184002941108282.jpg)
